Gram Staining- Principle, Reagents, Procedure, Steps, Results

It is the most widely used and the most important staining technique in bacteriology, especially in medical bacteriology. It is generally the first test performed on bacteria during their identification and observation process.
This staining technique uses two stains; crystal violet as primary stain and safranine as a counterstain. Those bacteria with Gram-positive cell walls will retain primary stain and appear violet or purple. These bacteria are termed Gram-Positive bacteria.
The other group of bacteria with Gram-Negative cell wall will lose primary stain and take up the counterstain and appears pink or red under the microscope. These bacteria are called Gram-Negative bacteria. Using this staining technique, bacteria can be differentiated into two groups hence; it is called the differential staining technique.

This technique was introduced in 1884 by the Danish Bacteriologist Hans Christian Gram (1853 September 13 to 1938 November 14). He developed this staining technique to identify bacteria causing pneumonia. Later it became a popular method to classify bacteria into Gram Positive and Gram Negative types.
Gram Staining Objectives
- To differentiate bacteria into Gram-Positive and Gram-Negative.
- To study the morphological structure of bacteria.
Gram Staining Principle
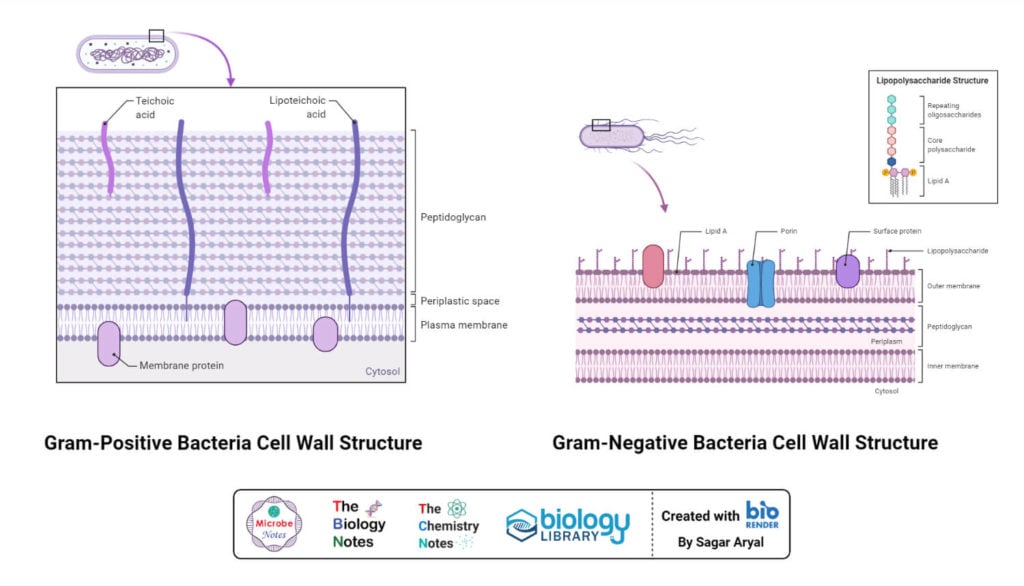
Gram staining and differentiation are based on the differences in cell wall structure and composition of bacteria. Bacteria having cell walls with a thick layer of peptidoglycan will resist decolorization of primary stain and appear violet or purple. Bacteria having a thin peptidoglycan layer with lesser cross-linkage lose primary stain during decolorizing and gain counter stain appearing pink or red.
In an aqueous solution of crystal violet dye, their molecules dissociate into CV + and Cl – ions. These ions easily penetrate the cell wall components of both positive and negative bacteria. The CV + ion interacts with negatively charged components of the cell wall. When Gram’s Iodine is added as mordant, the iodine (I – or I -3 ion) interacts with CV + ion and forms CV-I complex within cytoplasm and cell membrane and cell wall layers.
When decolorizing solution (ethanol or a mixture of ethanol and acetone) is added it interacts with lipids in the cell wall. The outer membrane of the Gram-Negative bacterial cell wall is dissolved exposing the peptidoglycan layer. The peptidoglycan layer is thin with less cross-linking in the Gram-Negative cell wall, hence becoming leaky. This causes cells to lose most of the CVI complexes. Whereas in Gram-Positive bacteria, there is no outer membrane, and the peptidoglycan layer is also thick with higher cross-linkage. So, the decolorizing solution dehydrates the peptidoglycan layer trapping all the CVI complexes inside the cell wall and bacteria retain the purple or violet color of crystal violet.
When counterstain, positively charged safranin, is added, it interacts with the free negatively charged components in Gram-Negative cell wall and membrane and bacteria becomes pink/red. Whereas, there is no space to enter inside the dehydrated Gram-Positive cell wall due to CVI complex and dehydration. Hence, safranin can’t stain them red or pink and Gram-Positive bacteria reveal the purple or violet color.
Gram Staining Requirements
- Sample bacterial colonies or suspension
- Gram Staining Kit (Reagents)
- Glass slide
- Inoculating loop
- Bunsen burner
- Staining rack
- Wash bottle (or Tap water)
- Microscope with 100X objective lens (compound microscope)
Gram staining procedure uses different chemicals and dyes that can be grouped;
1. Primary Stain (Crystal Violet)
It is an intensely purple-colored organic compound chemically called triphenylmethane dye. It is also known as hexamethyl pararosaniline chloride or methyl violet 10B or gentian violet. Its color depends on the pH of the dissolving medium such as, at pH -1.0 or below, it appears yellow, and at acidic pH of 1 to 2 it appears green, at neutral pH, it appears purple (deep blue-violet), and at highly basic pH it appears colorless.
It is used for staining textiles, papers, and fibers, in ball pens, and chemicals like detergents, fertilizers, etc.
In microbiology and molecular biology, it is used for staining bacteria, histological slide staining, DNA staining, etc. It also shows antibacterial and antifungal properties, hence used in sterilization and disinfection.
In Gram Staining, it is used as a basic dye in the ionized form of CV+ and Cl-. It provides violet color to Gram-Positive bacteria.
2. Mordant (Gram’s Iodine)
It is an aqueous solution of iodine and potassium iodide used as mordant in Gram staining. It interacts with CV+ and forms a CVI complex which gets trapped in the dehydrated peptidoglycan layer of the Gram-Positive cell wall.
3. Decolorizing Solution
It is either acetone or ethanol (95%) or a mixture of acetone and ethanol in ratio 1:1 by volume. The decolorizing solution dissolves the lipid content in the outer membrane of the Gram-Negative cell wall and increases its permeability. Whereas, in the Gram-Positive cell wall the decolorizer dehydrates the peptidoglycan layer and traps the CVI complex within the cell.
4. Counter Stain (Safranin)
It is a red-colored counterstain used to stain decolorized Gram-Negative cells in the Gram Staining technique. It is a basic dye that interacts with negatively charged components of the cell wall and membrane.
Besides safranin, dilute carbol fuchsin solution is also used as a counterstain.
Procedure of Gram Staining
Gram Staining Reagents Preparation
- Crystal Violet Preparation
- For preparing solution A (Crystal Violet stock solution), add 20 gm of 85% crystal violet dye in 100 ml ethanol (95%) and dissolve by mixing thoroughly.
- For preparing solution B (Oxalate Stock solution), add 1 gm ammonium oxalate in 100 ml distilled water and dissolve by mixing thoroughly.
- For preparing the working solution, add 1 ml of crystal violet stock solution in 10 ml distilled water and add 40 ml of oxalate stock solution.
- Let the solution sit for 24 hours at room temperature
- Store the solution in a dark bottle for use.
- Gram’s Iodine Preparation
- Dissolve 1 gm of Iodine, and 2 gm of potassium iodide in 300 ml distilled water.
- Mix properly till the iodine dissolve and keep the solution in a dark bottle.
- Decolorizing Solution
- Mix 50 ml of acetone with 50 ml of 95% ethanol
- Safranin
- Mix 2.5 gm of safranin-O in 100 ml of 95% ethanol
- Mix 10 ml of the above solution with 90 ml of distilled water to prepare a working solution
- Carbol-fuchsin
- Dissolve 3 gm of basic fuchsin in 100 ml of 95% ethanol
- Mix 5 ml liquid phenol with 95 ml of distilled water to prepare 5% phenol solution
- Mix 10 ml of basic fuchsin solution with 100 ml of 5% phenol solution
- Let the solution sit for 24 hours at room temperature
- Store in a dark bottle
Procedure of Gram Stain Slide Preparation
- Take a clean, clear, grease-free glass slide
- Sterilize the inoculating loop by flaming and transfer a loop full of bacterial culture suspension in the middle of the glass slide.
If culture is on a petri dish or slant, place a drop of water in the middle of the glass slide and using a sterile loop, transfer a small amount of colony and suspend with the water drop.
- Spread the suspension with the sterile inoculating loop to prepare a thin smear. The smear must not be too thin or too thick.
- Let the smear air dry and fix it by passing over the flame. Fixing should be done over a gentle flame. Slide must be moved up and down or circularly over the flame to prevent from overheating. Flaming will fix the bacterial cells on the slide and prevent them from washing out.
Gram Staining Protocol
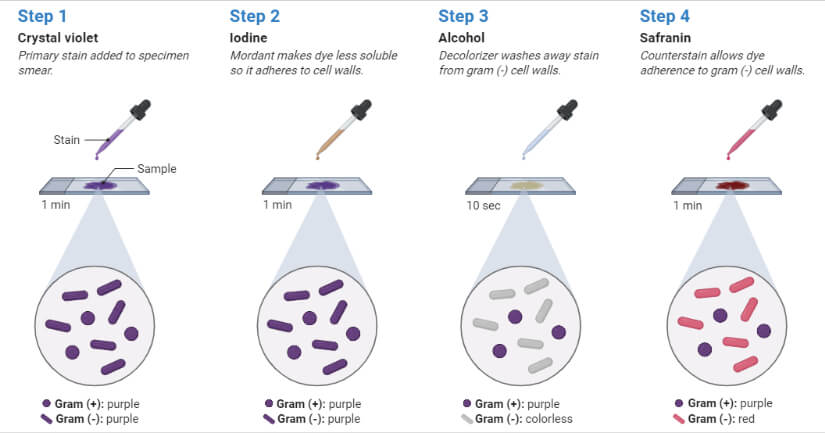
- Flood crystal violet solution over fixed smear
- After 30 – 60 seconds, pour off the CV solution and rinse with gentle running water.
- Flood the Gram’s Iodine solution over the smear
- Leave the iodine solution for 30 – 60 seconds and pour off the excess iodine and rinse with gentle running water
- Shake off the excess water over the smear
- Decolorize the smear by passing the decolorizing solution till the solution runs down in clear form. Alternatively, add a few drops of decolorizing solution and shake gently and rinse with distilled water after 5 seconds.
- Rinse with distilled water to wash decolorizer
- Shake off the excess water over the smear
- Pour counter stain over the smear
- Leave for 30 – 60 seconds and wash with gentle running water
- Air dry or blow-dry the smear.
Procedure of Microscopic Observation of Gram Stain
- Place the slide with Gram-stained air-dried smear over the stage of a compound microscope and fix it with stage clips
- Move the stage to focus light over smear
- Align the 10X objective lens and focus the smear using a coarse adjustment knob
- Change the objective lens to 40X and focus using the fine adjustment knob
- Rotate the nose piece so that the smear fall between 40X and 100X objective
- Add a drop of immersion oil over the smear
- Rotate the nose piece so that the 100X oil immersion objective lens is aligned over the smear
- Focus the microscope using a fine adjustment knob and study the bacteria
Result and Interpretation of Gram Staining
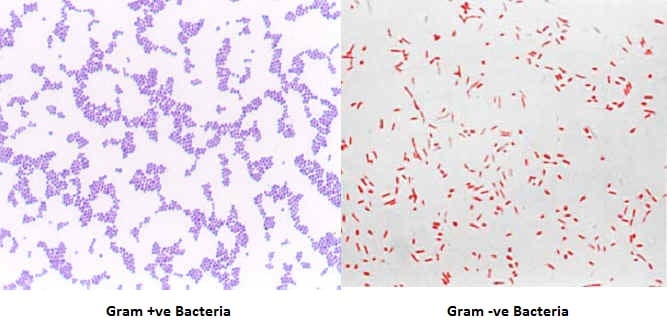
Examples of Gram-positive bacteria:
- Gram-positive cocci– Staphylococcus spp., Streptococcus spp., Enterococcus spp., etc.
- Gram-positive bacilli– Bacillus spp., Clostridium spp., Lactobacillus spp., Streptomyces spp. and other Actinobacteria, Listeria spp., Corynebacterium spp., etc.
Examples of Gram-Negative bacteria:
- Gram negative cocci– Neisseria spp., Moraxella spp., Acinetobacter spp. etc
- Gram negative bacilli-E. coli, Klebsiella spp., Salmonella spp., Shigella spp., Pseudomonas spp., Proteus spp., etc.
Applications of Gram Staining
- Used in research to classify the bacteria into Gram-positive and Gram-negative
- Used in diagnostic labs for identification of the pathogen
- Used in hospital for choosing spectrum of antibiotic for treatment before complete identification of bacteria
- Used to study the morphology of bacteria
Limitations of Gram Staining
- Can’t stain Acid Fast Bacilli (Mycobacterium spp.,), and bacteria without cell wall like Mycoplasma spp.
- Unsuitable for minute bacteria like Ricktessia spp., Chlamydia spp., etc.
- Require multiple reagents.
- Over-decolorization may result in the identification of false gram-negative results, whereas under-decolorization may result in the identification of false gram-positive results.
- Smears that are too thick or viscous may retain too much primary stain, making the identification of proper Gram stain reactions difficult. Gram-negative organisms may not decolorize properly.
- Cultures older than 16 to 18 hours will contain living and dead cells. Cells that are dead will be deteriorating and will not retain the stain properly.
- The stain may form a precipitate with aging. Filtering through gauze will remove excess crystals.
- Gram stains from patients on antibiotics or antimicrobial therapy may have altered Gram stain reactivity due to the successful treatment.
- Occasionally, pneumococci identified in the lower respiratory tract on a direct smear will not grow in culture. Some strains are obligate anaerobes.
- Toxin-producing organisms such as Clostridia, staphylococci, and streptococci may destroy white blood cells within a purulent specimen.
- Faintly staining Gram-negative organisms, such as Campylobacter and Brucella, may be visualized using an alternative counterstain (e.g., basic fuchsin).
References
- Thetner, D. (2000), “Triphenylmethane and related dyes”, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, doi:10.1002/0471238961, ISBN9780471484943.
- S013.pdf (himedialabs.com)
- Microsoft Word – IFU40168_MC_.doc (thermofisher.com)
- SAFRANIN: CHARACTERISTICS, USE, TECHNIQUES, TOXICITY – SCIENCE (warbletoncouncil.org)
- Bartholomew, J. W. and Finkelstein, H. (1958). Relationship of cell wall staining to Gram differentiation. J. Bacteriol., 75(1): 77-84.
- Gram Stain: Introduction, Principle, Procedure, Result and Interpretation (universe84a.com)
- Gram stain | Principle | Steps | Interpretation | Tips (microbiologie-clinique.com)
- Tripathi N, Sapra A. Gram Staining. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
- Gram Staining : Principle, Procedure, Interpretation and Animation – Laboratoryinfo.com
- Gram Staining: Principle, Procedure, Interpretation, Examples and Animation (microbiologyinfo.com)
- Gram Staining: Principle, Procedure, Results • Microbe Online
- Preparation of Gram stain Reagents • Microbe Online
- Microsoft Word – Gram Staining Protocol v3 (goldbio.com)
About Author
Prashant Dahal completed his bachelor’s degree (B.Sc.) Microbiology from Sunsari Technical College, affiliated with Tribhuvan University. He is interested in topics related to Antimicrobial resistance, the mechanism of resistance development, Infectious diseases (Pneumonia, tuberculosis, HIV, malaria, dengue), Host-pathogen interaction, Actinomycetes, fungal metabolites, and phytochemicals as novel sources of antimicrobials and Vaccines.






